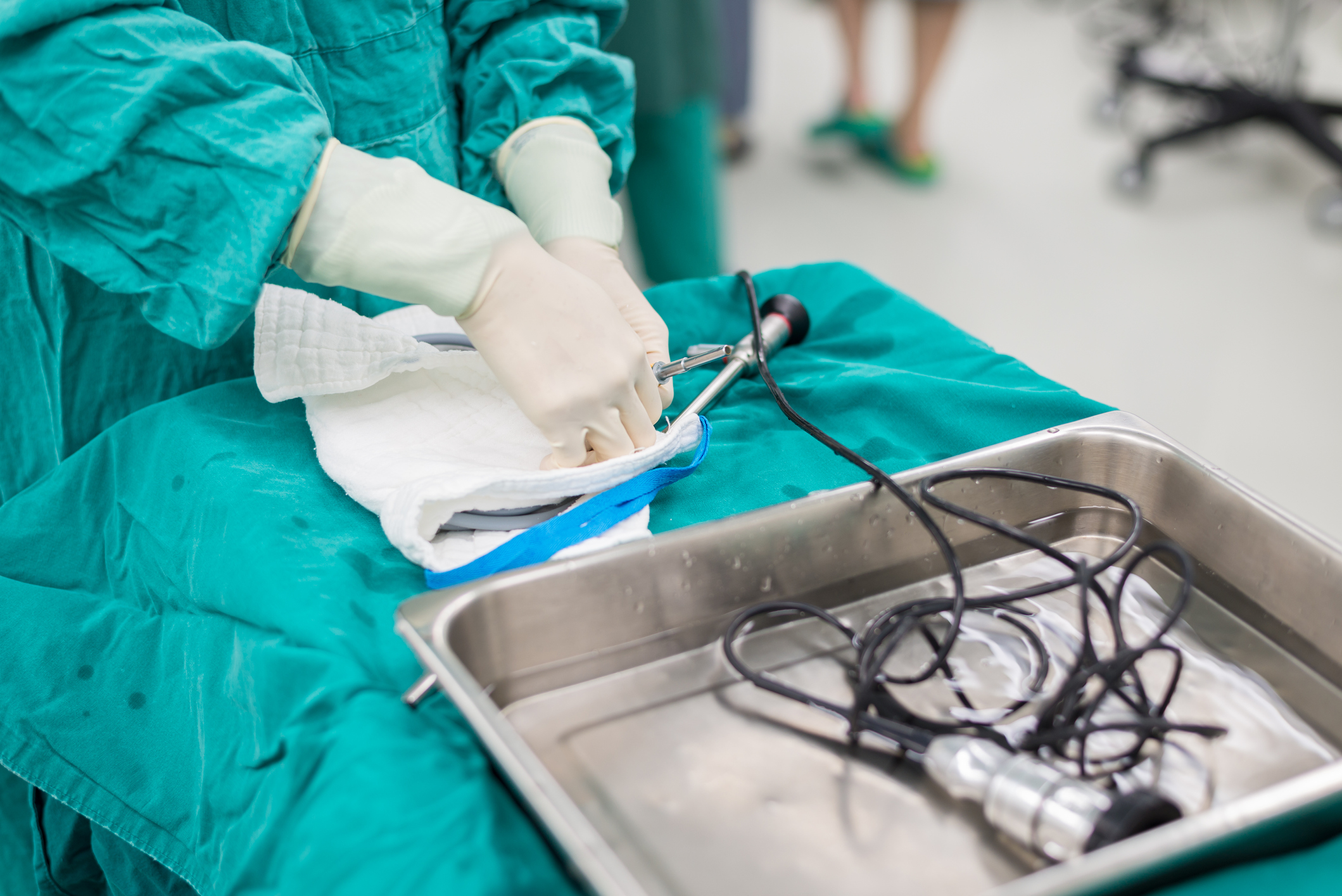
A recent article in The New York Times examines the difficulty healthcare providers face in properly sterilizing duodenoscopes.
The article — "Why Are These Medical Instruments So Tough to Sterilize?" — begins by noting the tremendous value of using duodenoscopes. It then describes the unusual and sometimes unsatisfactory cleaning process these devices must undergo. When this process comes up short, causing a duodenoscope to retain bacteria, patient safety is jeopardized. Numerous patients have become ill from contracting infections — including antibiotic-resistant infections — from dirty duodenoscopes.
As a result, the article notes, some medical experts have reached out to the U.S. Food and Drug Administration (FDA), pressing the agency to either require manufacturers to develop duodenoscopes that can be properly sterilized or take them off the market. Supporting this request is data that recently showed one in 20 duodenoscopes retained bacteria after undergoing proper cleaning processes.
In the piece, UNC Health Care's Dr. David Jay Weber, medical director for UNC, is quoted as saying, "Would you go on an airplane if the pilot said, 'By the way, there is a 5% chance the engines will fail'? Would you go in a car if the manufacturer said, 'There are airbags, but 5% of the time they won't deploy'?"
Infection Control Consulting Services (ICCS) regularly draws attention to duodenoscope infection prevention challenges. While duodenoscopes are designed differently from colonoscopes and gastroscopes, performing appropriate high-level disinfection that comply with manufacturers' instructions for use (IFUs) and nationally recognized guidelines remains critical for these scopes as well. Any shortcomings in following IFUs and guidelines can significantly increase infection risks. If your organization requires assistance with central sterile processing, learn how ICCS can help.